Company Overview
ThinSpring Takes You To The Future of Structured Content
Information Technology Solutions For Medical Products Built By Medical Product
Professionals
ThinSpring management has decades of experience in pharmaceutical and medical products manufacturing. We provide a deep understanding of the multitude of regulatory requirements and market challenges facing your organization. Our business solutions are designed to meet the challenges of today while providing a platform for future growth.
| ThinSpring's Structured Content Management Solution |
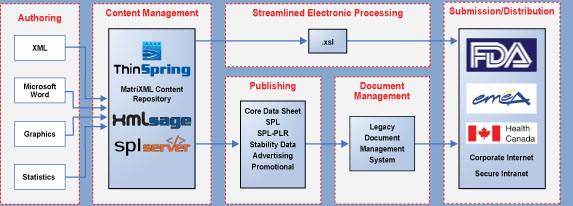
|
Consideration For Now & The Future
ThinSpring's solution set provides value now and for the future of structured content management.
- Full Range of Structured Content Management Solutions including SPL, eCTD, ICSR and eMDR
- FDA Solution Provider
- Corporate Leaders from Careers in Life Sciences
- World’s Leading Provider of SPL Solutions
ThinSpring’s Competitive Edge
Through revolutional technology, ThinSpring is the only provider in the industry that can offer the full breadth of structured content solutions.
ThinSpring products & services include:
XML Sage™ - Our flagship product that supports your company’s full submission life cycle by providing you the capability to create, update, store and publish the complete set of product dossier documents.
SPL Server™ - The industry-leading SPL solution that gives you the power to create, edit and submit fully validated submissions every time. You choose between native structure content authoring and Rich Text Editing.
Online SPL Validation - Did you know that the FDA recently announced that over 25% of SPL submissions to date have been rejected due to Tier I and Tier II validation errors? When you use any of ThinSpring’s solutions you can be 100% confident that you will never receive an RTF for this.
SPL/PLR Conversion - Get support for your SPL conversion needs every step of the way from initial conversion and FDA negotiations through final delivery of SPL content. This includes ongoing industry alerts and updates as well as structured content education & training.
Rich Text Editor Authoring - Use the Rich Text Editor interface to author, delete main sections, sub-sections, paragraphs, tables, ordered lists, unordered lists, and notes.
SPL-PLR Medical Terminology - We offer services to assist sponsors meet the challenges of the PLR guidelines.
Human Language Translation - You can have all of your translation needs met seamlessly across all countries, regulatory bodies, and technologies.
Why ThinSpring?
Improve Time to Market - Risk is minimized with no refusals to file or content being flagged for additional reviewer scrutiny. Our solutions are built from the ground up using our solid experience in providing structured content management solutions to corporations in the life sciences industry.
We know first-hand the pressures of day-to-day operations as you work to comply with regulations that are constantly changing. We prove every day that compliance and profit are not mutually exclusive. Flexible pricing model fits capital and expense budget constraints enabling departmental, facility and enterprise solutions.
IT Infrastructure Ready
A true thin client approach to structured content solutions reduces implementation time and IT maintenance costs. Open standards support make ThinSpring solutions extremely cost-effective by limiting the dollars you are required to spend on hardware & software.
Database administration tasks are minimized as XML is dynamically mapped to and from the relational database management system repository. Our XML infrastructure technology addresses medical product master file solutions throughout the product life cycle.
Our tools are not just answers to today’s challenges, they are solutions for the future. XML is rapidly becoming the de facto standard for electronic file interchange. ThinSpring’s solutions allow native XML authoring and full medical product life cycle management now. ThinSpring solutions meet XML web standards, electronic signature and records management compliance now. No retrofitting is required.
Our cutting edge technology is supported by our own subject matter experts to deliver superior solutions to you!